
Aerosols are known as pressurized packages, which us liquified propellants. These are convenient and easy to use. Medication is dispensed in a ready-to-use form on pressing a button
Aerosol is defined as a dispersion system that functions on the power of compressed or liquified gas to expel the contents from the container.
Aerosol is defined as a dispersion system that functions on the power of compressed or liquified gas to expel the contents from the container.
Some aerosol products in the market with their uses are given below:
- Local anesthetic: Heales spray(Ayurvedic Products)
- Antiasthmatic: Beclomethasone dipropionate
- Analgesic: Iodex spray
- Bronchodilatory agents: salbutamol spray
- Antihistaminic: Azelastine hydrochloride nasal spray
Applications of Aerosols:
- Administration of drugs from aerosol products is easy that's why it can be applied directly on the affected parts or abraded skin introduced into body cavity & passages.
- Aerosol administration gives very efficient & quick relief.
- When sprayed on skin, some of propellants (Ethyl chloride) cool the tissue due to sudden expansion of propellant.
Mechanism & Function of aerosol:
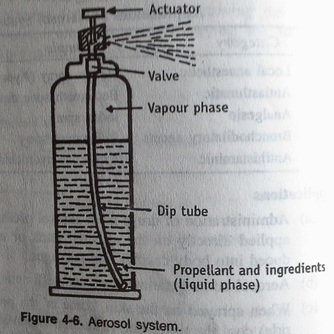
The assembly of aerosol system is shown in diagram:
Components of aerosols are:
- Product concentrate :
Function: Drug with other ingredients.
Types: Solution, suspension or emulsion.
- Container:
Function: To carry the product.
Types: Tin-plated, steel or glass.
- Propellant:
Types: Trichlorofluoromethane, dichlorodifluoromethane are most commonly use.
- Valve:
Types: Continuous spray valve(when to press the button, it will continuously spray the medicine/liquid), Metering valve ( when you press the button, it will spray some amount of medicine).
- Actuator:
Function: For opening the valve and producing requirements types of discharge.
Types: Sprays & foams.
The product concentrate in the container is in equilibrium between liquid & gaseous state. When actuator is pressed the valve opens. Since the product is under pressure, the vapour above the liquid concentrate pushes the products down. Then the product get expelled through dip-tube from the container
The compressed gas(propellent) should have initial pressure(usually 550 KPa) & remain constant during usage. The required calculation in the usage of gases can be done using Boyle's law & ideal gas equation.
Production of Aerosol products:
Two methods are employed in production of aerosols:

Pressure filling method:
In this method, the products concentrate is placed in the container and closed with the valve. The product is maintained below Critical temperature/slightly below boiling point(Critical temperature is defined as the temperature above which the liquid can no longer exist as liquid or in easy term, temperature above which liquid show properties which are intermediate between gas & liquid). The propellant gets liquified in the container.
In this method, the products concentrate is placed in the container and closed with the valve. The product is maintained below Critical temperature/slightly below boiling point(Critical temperature is defined as the temperature above which the liquid can no longer exist as liquid or in easy term, temperature above which liquid show properties which are intermediate between gas & liquid). The propellant gets liquified in the container.
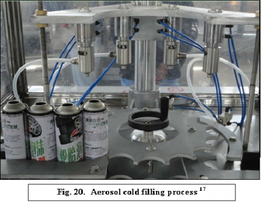
Cold filling method:
This method involves the conventional process of filling. Since the propellant is a gas at room temperature, it is liquified by lowering the temperature below its critical temperature(critical pressure is defined as the pressure required to liquefy a gas at its critical temperature). The product Concentration is also cooled to the temperature of about -30°c to - 40°c. The chilled concentrate is introduced into the chilled container and liquefied propellant is added. Sufficient time is given for propellent to get vapourized partially in order to expel the air present in the container. The valve is fitted & the container is closed. After packaging, the container is equilibrated to the room temperature. The propellent in the container gets vaporized partially and build up sufficient pressure. Once the valve is opened the contents are expelled due to high pressure in container
This method involves the conventional process of filling. Since the propellant is a gas at room temperature, it is liquified by lowering the temperature below its critical temperature(critical pressure is defined as the pressure required to liquefy a gas at its critical temperature). The product Concentration is also cooled to the temperature of about -30°c to - 40°c. The chilled concentrate is introduced into the chilled container and liquefied propellant is added. Sufficient time is given for propellent to get vapourized partially in order to expel the air present in the container. The valve is fitted & the container is closed. After packaging, the container is equilibrated to the room temperature. The propellent in the container gets vaporized partially and build up sufficient pressure. Once the valve is opened the contents are expelled due to high pressure in container
-By Devesh Chaudhari
Thank you for reading my Article.
 RSS Feed
RSS Feed

